Our innovation encompasses the development of novel therapeutic treatments based on an analysis of isotopic signatures in the cells and tissues affected by different pathologies. In an era of advanced drug discovery when researchers exploit molecular signatures, we are taking our research further into atomic level to study the effects of isotopic fractionation of essential chemical elements that play important roles in biological sequences.
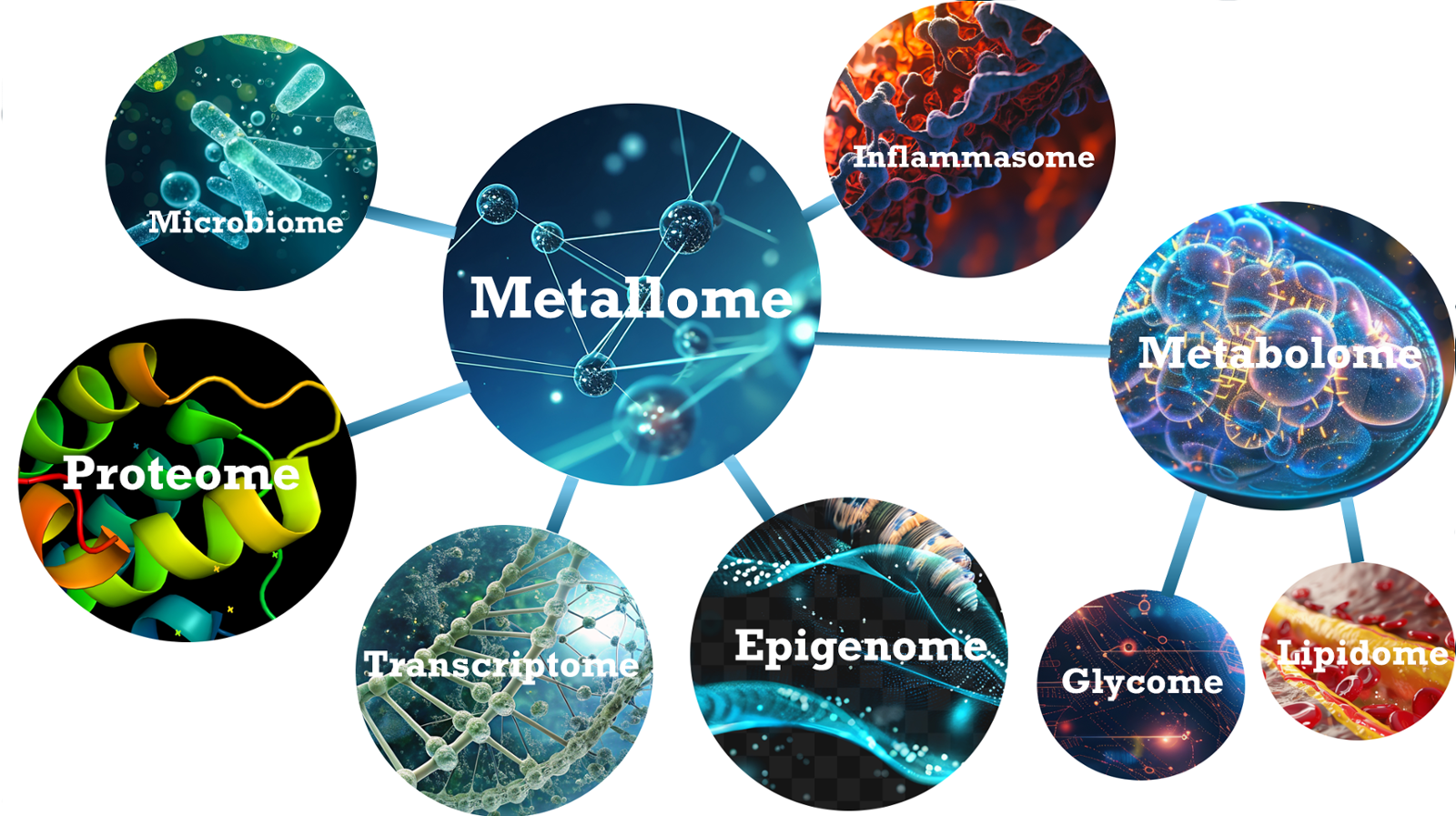
Metallome connects the "Omes"...
The metallome encompasses the distribution of metal ions within tissues and cells, and is critical for a myriad of biological processes, including the synthesis of amino acids and proteins, maintaining charge balance and electrolyte function, ensuring DNA integrity and facilitating its repair, as well as playing a key role in structure, signaling, and stem cell functionality. Our products are designed to stimulate the fundamental biological activities of the metallome to re-establish a state of healthy homeostasis.
Taking the Science of Metallomics to the Subatomic Level!
ZnA Oncology has emerged to revolutionize the metallomics approach to oncology with our distinctive scientific perspective on disease pathogenesis. Our approach is rooted in over ten years of research that delves beyond molecular signatures, further into atomic and nucleus levels to explore the isotopic signatures as part of metalloproteomics. Our first-in-class therapeutic formulation, KLS1, is based of light isotope 64Zn enriched >99%.
Focus on p53 Protein
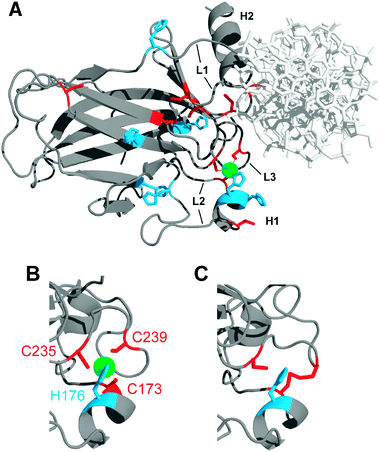
The p53 protein is known as the "guardian of the genome" because it helps to protect cells from damage and is the key part of the body's defense against cancer. Zinc plays critical role in creating and maintaining the proper structure and function of p53. This relationship is particularly important in the context of cancer, where mutations in p53 cause loss of lives.
The wild type 53 protein (wtp53) requires the binding of a single zinc ion for proper folding and function. This zinc ion thereby is coordinated by four specific amino acids within the DNA-binding domain (DBD) of the protein: Cysteine 176; Cysteine 238; Cysteine 242; and Histidine 179. Three of these specific amino-acids, Cysteine (S-ligands), preferentially binds isotopically light Zn, while normally tighter binding histidine (N-ligands) and aspartate (O-ligands) preferentially complex isotopically heavy Zn (ref.)
Our studies show that tumors are prevalent heavy zinc isotopes.
Harnessing Cold Isotopes for Multi-Omic Health
Elevated levels of heavy isotopes significantly interfere with several essential cellular functions and play a major role in inducing both inflammation and oxidative stress, which are critical precursors to the development of various diseases. Our isotope-selective modulation approach explores how metal isotopes impact these biological functions and disease cascades. By utilizing light cold isotopes, we are developing safe and effective drugs that engage multiple pathways for enhanced therapeutic outcomes in treating complex diseases and can be used in patients with many oncological illnesses.
The Role of Ageing
The Role of Ageing
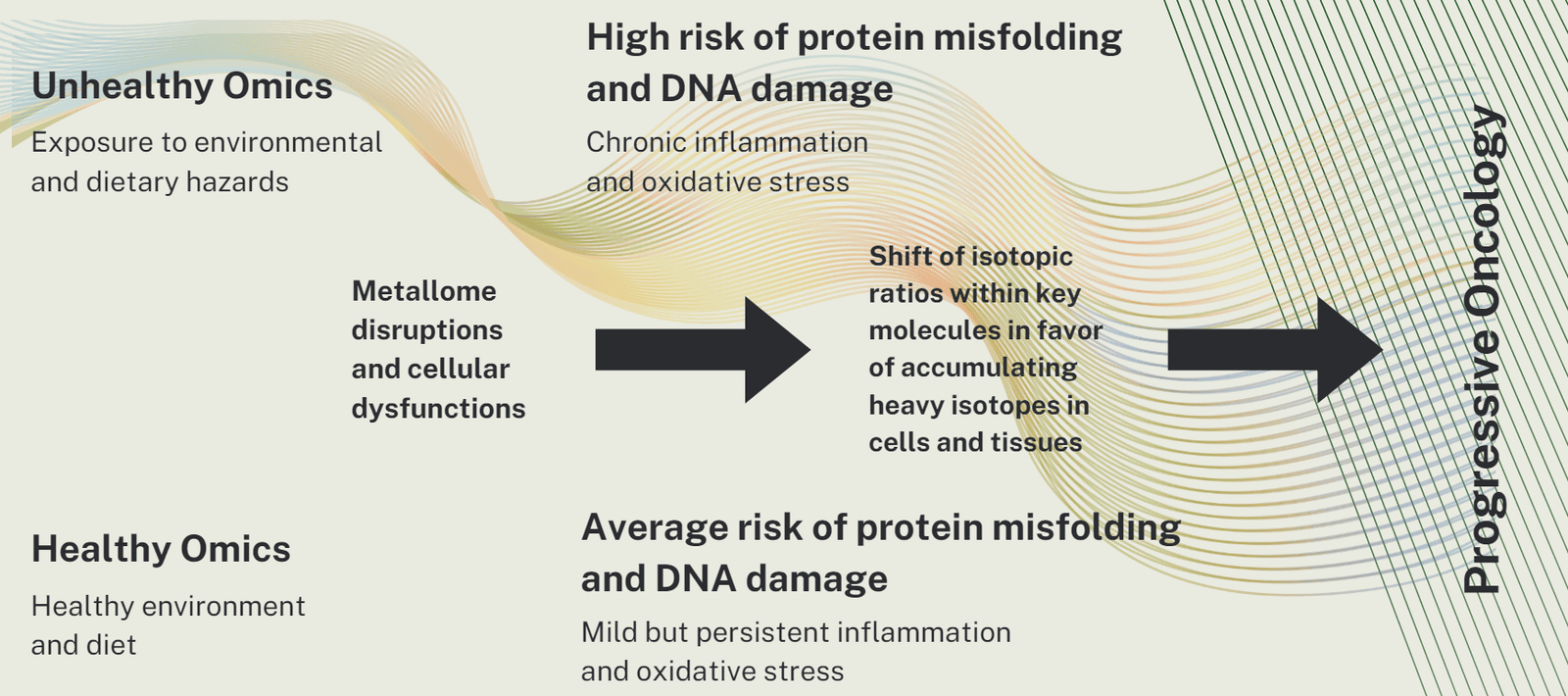
Unhealthy aging, when combined with exposure to dietary and environmental hazards, can significantly exacerbate the imbalance of various isotopes in the body, thereby accelerating the progression of various pathologies. This complex interaction involves several mechanisms, primarily through the disruption of metal homeostasis and the subsequent biological responses that can lead to disease.
Metal isotopes, such as iron, copper, zinc, and calcium, play crucial roles in numerous biological processes, including enzyme function, oxygen transport, and cellular signaling. As we all age, the regulatory systems that maintain the balance of these isotopes become less efficient. Environmental and dietary factors can also introduce heavy metals like lead, mercury, and cadmium into the body, which are known to compete with essential metals, disrupting their homeostasis.
Pathological Consequences of Isotope Imbalance
Pathological Consequences of Isotope Imbalance
The imbalance of metal isotopes can lead to oxidative stress, a condition where harmful free radicals overwhelm the body's antioxidant defenses. This oxidative stress is a key factor in the development of chronic diseases such as cardiovascular disease, diabetes, and cancers. Additionally, certain heavy metals can directly induce cellular toxicity, leading to apoptosis or necrosis, and can alter DNA methylation patterns, which are crucial for regulating gene expression and maintaining genomic stability.
Implications for Disease Progression
Implications for Disease Progression
We believe that a disease is an "acute local ageing". The disruption of metal isotope homeostasis can accelerate the local aging process, further compounding the risk of age-related diseases. This is because aging itself is associated with a natural decline in the body's ability to repair and maintain cellular and systemic integrity. When coupled with isotope imbalance, the physiological stresses on the body is amplified, leading to an increased incidence and severity of diseases.